lithium number of neutrons|Chemistry of Lithium (Z=3) : Tuguegarao Mar 23, 2023 Find all the best Cheltenham free bets for the 2024 Festival.. Sky Bet ITV7. Sky Bet runs the ITV7 on every day of the Cheltenham Festival, giving both new and existing customers the chance to win .
lithium number of neutrons,The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. .
Lithium is a soft, silvery-white alkali metal with atomic number 3 and neutron number 6. The web page provides detailed information about the properties of lithium, . Mar 23, 2023
The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, .lithium number of neutrons Chemistry of Lithium (Z=3) The 6 Li isotope is one of only five stable nuclides to have both an odd number of protons and an odd number of neutrons, the other four stable odd-odd nuclides being hydrogen-2, boron-10, nitrogen-14, and tantalum .

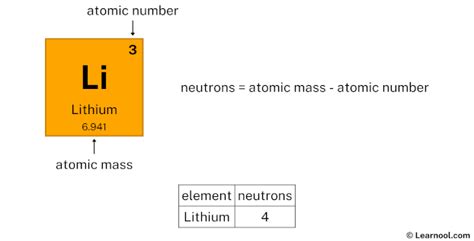
Lithium is the 3rd element in the periodic table and has a symbol of Li and atomic number of 3. It has an atomic weight of 6.940 and a mass number of 7. Lithium has three protons . Lithium is an alkali metal with the atomic number = 3 and an atomic mass of 6.941 g/mol. This means that lithium has 3 protons, 3 electrons and 4 neutrons (6.941 - .Lithium atom. A lithium atom is an atom of the chemical element lithium. Stable lithium is composed of three electrons bound by the electromagnetic force to a nucleus containing .PubChem CID Number. 3028194. Lithium is a chemical element of the periodic table with chemical symbol Li and atomic number 3 with an atomic weight of 6.938 u and is classed .Lithium (Li) Lithium is the 3rd element in the periodic table and has a symbol of Li and atomic number of 3. It has an atomic weight of 6.940 and a mass number of 7. Lithium has three protons and four neutrons in its nucleus, and three electrons in two shells. It is located in group one, period two and block s of the periodic table. Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (+1) ( + 1) and a mass of 1 atomic mass unit (amu) ( amu), which is about 1.67 ×10−27 1.67 × 10 − 27 kilograms. Together with neutrons, they make up virtually all of the mass of an atom. Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Cite this Article. . All atoms of the same element have the same number of protons, but some may have different numbers of neutrons. For example, all carbon atoms have six protons, and most have six neutrons as well. . Notice that because the lithium atom always has 3 protons, the atomic number for lithium is always 3. The mass number, however, is 6 in .

Because the number of neutrons can vary for a given element, the mass numbers of different atoms of an element may also vary. For example, some helium atoms have three neutrons instead of two (these are called isotopes and are discussed in detail later on) . the atomic number for lithium is always 3. The mass number, however, is 6 .
Atomic Number of Lithium. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure. The chemical symbol for Lithium is Li. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons . When the numbers of these subatomic particles are not equal, the atom is electrically charged and is called an ion. The charge of an atom is defined as follows: Atomic charge = number of protons − number of electrons (1.8.1) (1.8.1) Atomic charge = number of protons − number of electrons.All atoms of the same element have the same number of protons, but some may have different numbers of neutrons. For example, all carbon atoms have six protons, and most have six neutrons as well. . In a chunk of lithium, \(93\%\) will always be lithium with 4 neutrons, while the remaining \(7\%\) will always be lithium with 3 neutrons.The mass number is defined as the total number of protons and neutrons in an atom. It can be calculated by adding the number of neutrons and the number of protons (atomic number) together. Mass number = atomic number + number of neutrons. Consider Table 4.17.1 4.17. 1 below that shows data from the first six elements of the periodic table.In this case, hydrogen (H) has an atomic number of 1 and, therefore, every atom of hydrogen will contain 1 proton. The equation shown above can then be applied, as follows. Mass Number = # of Protons + # of Neutrons. Mass Number = 1 + 2. Therefore, this particular atom of hydrogen will have a mass number of 3. All atoms of the same element have the same number of protons, but some may have different numbers of neutrons. For example, all carbon atoms have six protons, and most have six neutrons as well. . In a chunk of lithium, \(93\%\) will always be lithium with 4 neutrons, while the remaining \(7\%\) will always be lithium with 3 neutrons.The 6 Li isotope is one of only five stable nuclides to have both an odd number of protons and an odd number of neutrons, the other four stable odd-odd nuclides being hydrogen-2, boron-10, nitrogen-14, . Although .
All atoms of the same element have the same number of protons, but some may have different numbers of neutrons. For example, all carbon atoms have six protons, and most have six neutrons as well. . Notice that because the lithium atom always has 3 protons, the atomic number for lithium is always 3. The mass number, however, is 6 in the . Make sure that you round the atomic mass to the nearest whole number. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11. 6. Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass.Name of the isotope: Lithium-7; Li-7 Symbol: 7 Li or 73 Li Mass number A: 7 (= number of nucleons) Atomic number Z: 3 (= number of protons) Neutrons N: 4 Isotopic mass: 7.01600344 (3) u ( atomic weight of Lithium-7) Nuclide mass: 7.0143577 u (calculated nuclear mass without electrons) Mass excess: 14.90711 MeV Mass defect: . In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Lithium (LI). From the Periodi.This is because the mass of the proton and neutron are each about 1 amu, while the mass of the electron is very small in comparison. mass number(A) = number of protons + number of neutrons (4.7.1) (4.7.1) mass number ( A) = number of protons + number of neutrons. Consider oxygen, which has an atomic number ( Z Z) of 8.
lithium number of neutrons Figure 3.4.1 3.4. 1: The social security number subatomic-the proton. Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Helium, with two protons, will have two electrons.
lithium number of neutrons|Chemistry of Lithium (Z=3)
PH0 · Protons, Neutrons, Electrons for Lithium(Li, Li+)
PH1 · Protons Neutrons & Electrons of All Elements (List
PH2 · Lithium atom
PH3 · Lithium (Li)
PH4 · Lithium
PH5 · Chemistry of Lithium (Z=3)